Breakthrough Xenotransplant Study Reveals Immune Response in Pig-to-Human Kidney Transplant
A groundbreaking Nature study published in November 2025 provides unprecedented insights into the immune dynamics of pig-to-human kidney xenotransplantation. Researchers conducted multi-omics profiling over 61 days in a decedent human recipient, revealing detailed immune cell interactions and rejection mechanisms. The study identifies specific immune cell populations, including plasmablasts, NK cells, and dendritic cells that increased during early rejection phases, while T-cell responses emerged later. These findings offer crucial understanding of xenotransplant rejection and potential immunomodulatory targets to improve future transplant success rates.
Organ transplantation faces a critical shortage of available organs, with thousands of patients worldwide waiting for life-saving procedures. Xenotransplantation—the transplantation of organs between species—has emerged as a promising solution to this crisis. A groundbreaking study published in Nature provides unprecedented insights into the complex immune responses that occur during pig-to-human kidney transplantation, offering hope for future clinical applications.

Study Design and Methodology
The research team conducted large-scale multi-omics profiling of a pig-to-human kidney xenotransplant over a 61-day procedure in a brain-dead human recipient. This comprehensive approach allowed scientists to analyze the molecular orchestration of human immune responses to porcine kidney tissue in unprecedented detail. The study represents a significant advancement in understanding xenotransplant immunology and brings us closer to viable cross-species organ transplantation.
Immune Response Timeline
The research revealed a carefully orchestrated sequence of immune events following transplantation. Between postoperative days 10 and 28, researchers observed significant increases in blood plasmablasts, natural killer (NK) cells, and dendritic cells. This early immune activation coincided with expansion of IgG/IgA B-cell clonotypes, ultimately leading to biopsy-confirmed antibody-mediated rejection (AbMR) at postoperative day 33.
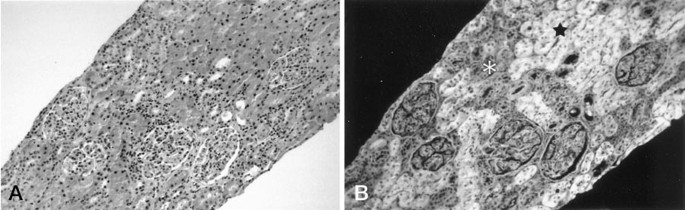
T-Cell Involvement and Combined Rejection
Human T-cell frequencies began increasing from postoperative day 21, peaking between days 33-49 in both blood and xenograft tissue. This T-cell response coincided with T-cell receptor diversification and expansion of a restricted TRBV2/J1 clonotype. The study documented histological evidence of combined antibody-mediated and cell-mediated rejection at postoperative day 49, highlighting the complexity of immune responses in xenotransplantation.
Macrophage Activity and Inflammation
At the peak of antibody-mediated rejection (postoperative day 33), the most abundant human immune population in the graft was CXCL9+ macrophages. This finding aligns with IFN-γ-driven inflammation and indicates a Type I immune response. The research also revealed evidence of interactions between activated pig-resident macrophages and infiltrating human immune cells, suggesting complex cross-species cellular communication.

Tissue Injury and Complement Activation
The xenograft tissue showed pro-fibrotic tubular and interstitial injury, marked by specific biomarkers including S100A6, SPP1 (Osteopontin), and COLEC11, between postoperative days 21-33. Proteomics profiling revealed both human and pig complement activation, with decreased human component following AbMR therapy with complement inhibition. This finding suggests potential therapeutic targets for managing rejection episodes.
Clinical Implications and Future Directions
This comprehensive multi-omics analysis provides crucial insights into the molecular mechanisms driving xenotransplant rejection. The identification of specific immune cell populations and their activation timelines offers potential immunomodulatory targets for improving xenograft survival. As researchers continue to refine gene-editing techniques and immunosuppressive strategies, these findings bring us closer to making xenotransplantation a viable solution to the organ shortage crisis.
The study represents a significant step forward in transplant medicine, demonstrating that while challenges remain, scientific understanding of xenotransplant immunology continues to advance rapidly. Future research building on these findings may eventually make cross-species organ transplantation a routine clinical practice, potentially saving thousands of lives annually.





