Cancer Cells' Hidden Defense: Mitochondrial Emergency Response to Physical Stress
Scientists have discovered a remarkable defense mechanism in cancer cells where mitochondria rush to the nucleus when cells are physically squeezed, unleashing an ATP surge that powers DNA repair and survival. This newly identified phenomenon, called nucleus-associated mitochondria (NAMs), reveals mitochondria acting as emergency first responders rather than static power plants. The discovery, visualized in real time using advanced microscopy, provides crucial insights into how cancer cells survive mechanical stress during metastasis and could lead to new therapeutic strategies to prevent cancer spread.
Cancer cells possess a remarkable hidden defense mechanism that activates when they face physical compression, according to groundbreaking research from the Centre for Genomic Regulation in Barcelona. When squeezed to just three microns wide—about one-thirtieth the diameter of a human hair—cancer cells immediately deploy mitochondria to the nucleus, creating a powerful energy surge that fuels DNA repair and enhances survival. This discovery fundamentally changes our understanding of cellular responses to mechanical stress and opens new possibilities for cancer treatment.
The Mitochondrial Emergency Response
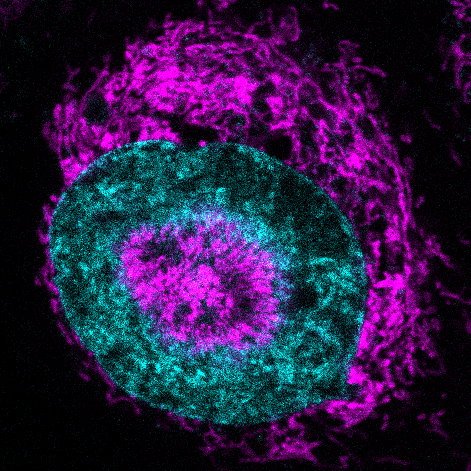
The research team observed this phenomenon using specialized microscopy that compresses living cells while monitoring their responses in real time. Within seconds of being squeezed, mitochondria in cancer cells race to the nuclear surface, forming tight halos that actually cause the nucleus to dimple inward. The researchers named these structures "NAMs"—nucleus-associated mitochondria—and observed them in 84 percent of confined cancer cells compared to virtually none in uncompressed cells.
This mitochondrial movement triggers a dramatic energy surge, with ATP levels in the nucleus increasing by approximately 60 percent within just three seconds of compression. As Dr. Sara Sdelci, co-corresponding author of the study published in Nature Communications, explains, "It forces us to rethink the role of mitochondria in the human body. They aren't these static batteries powering our cells, but more like agile first responders that can be summoned in emergency situations when cells are literally pressed to the limit."

Biological Significance and DNA Protection
The energy surge serves a critical protective function. Mechanical squeezing puts tremendous stress on DNA, causing strand breaks and tangling of the genome. Cells require substantial ATP to power repair mechanisms that can access and mend these damaged sites. The research demonstrated that cancer cells receiving this mitochondrial energy boost successfully repaired DNA damage within hours, while those without the NAM formation stopped dividing properly.
Dr. Fabio Pezzano, co-first author of the study, notes that "It's a clear sign the cells are adapting to the strain and rewiring their metabolism." This adaptive response helps explain how cancer cells survive the mechanical challenges they encounter during metastasis, including navigating through dense tumor microenvironments, entering blood vessels, and withstanding the turbulent forces of the bloodstream.
Clinical Relevance and Therapeutic Implications
The research team confirmed the real-world significance of their findings by examining breast tumor biopsies from 17 patients. They discovered that NAM structures appeared three times more frequently at invasive tumor fronts (5.4 percent of nuclei) compared to dense tumor cores (1.8 percent), suggesting this mechanism plays a role in cancer spread. Dr. Ritobrata Ghose, co-first author, emphasizes that "Seeing this signature in patient biopsies convinced us of the relevance beyond the lab bench."
The study also identified the cellular machinery enabling this mitochondrial response. Actin filaments and endoplasmic reticulum networks combine to create a scaffold that traps mitochondria at the nuclear periphery. When researchers disrupted this scaffold using latrunculin A, NAM formation collapsed and the protective ATP surge disappeared. This finding suggests potential therapeutic approaches that could target this mechanical stress response without broadly damaging mitochondria in healthy tissues.
Broader Biological Implications
While the study focused on cancer cells, the researchers believe this mechanism represents a universal biological response to physical stress. Immune cells squeezing through lymph nodes, neurons extending branches, and embryonic cells during development all experience similar mechanical forces that could trigger comparable mitochondrial responses. Dr. Sdelci concludes that "Wherever cells are under pressure, a nuclear energy boost is likely safeguarding the integrity of the genome. It's a completely new layer of regulation in cell biology, marking a fundamental shift in our understanding of how cells survive intense periods of physical stress."
This discovery not only enhances our fundamental understanding of cellular biology but also opens promising new avenues for cancer treatment by targeting mechanical stress responses that cancer cells depend on for survival and spread.