MAPK-Driven Cellular Plasticity: The Key to Colorectal Cancer Treatment Resistance
Groundbreaking research published in Nature reveals how MAPK signaling drives epithelial cell plasticity in colorectal cancer, creating therapeutic resistance to targeted treatments. The study demonstrates that oncogenic MAPK signaling induces regenerative stem-like populations, while inhibition triggers rapid transcriptional remodeling favoring Wnt-associated stem phenotypes. This cellular adaptability explains why KRAS and BRAF inhibitors often fail clinically, while identifying specific conditions where treatments succeed remarkably well.
Colorectal cancer treatment faces a significant challenge: the remarkable ability of cancer cells to adapt and resist targeted therapies. A groundbreaking study published in Nature reveals that MAPK-driven epithelial cell plasticity serves as the primary mechanism behind this therapeutic resistance, offering new insights into why some treatments fail while others succeed unexpectedly.

The MAPK Signaling Pathway in Colorectal Cancer
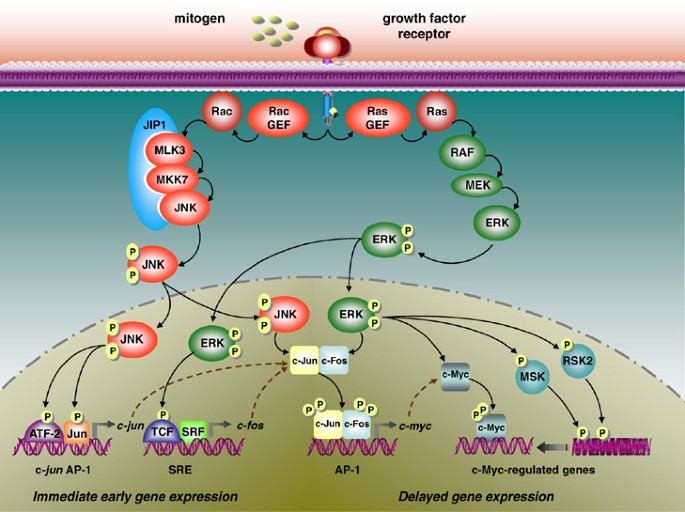
MAPK signaling represents a critical pathway in colorectal cancer development and progression. The research highlights that mutations in KRAS (occurring in 40-50% of cases) and BRAF (present in approximately 10% of patients) drive oncogenic MAPK signaling, making these pathways attractive targets for therapeutic intervention. However, the clinical reality has been disappointing, with resistance developing rapidly following treatment initiation.
Epithelial Cell Plasticity: The Resistance Mechanism
The study demonstrates that oncogenic MAPK signaling induces profound epithelial state changes in colorectal cancer cells. This cellular plasticity enables tumors to adopt regenerative, revival stem-like populations that can withstand therapeutic pressure. When MAPK inhibitors are administered, the cancer cells undergo rapid transcriptional remodeling, essentially reprogramming themselves to survive the treatment assault.
Differential Resistance Patterns
Researchers observed distinct resistance patterns between different genetic subtypes. Kras-mutant tumors developed acute therapeutic resistance, while Braf-driven models exhibited delayed resistance. This temporal difference suggests varying adaptive capabilities among different colorectal cancer subtypes, potentially informing timing and sequencing of treatment strategies.
Conditions for Therapeutic Success
The research provides crucial insights into when MAPK-targeted therapies actually work. When cellular plasticity is restrained, such as in early metastatic disease or through targeting specific genetic mutations like Rnf43 in the Wnt pathway, marked therapeutic responses occur. This explains the "super response" observed in BRAF/RNF43 co-mutant patient populations treated with BRAF+EGFR targeted therapies.
Clinical Implications and Future Directions
These findings have significant implications for colorectal cancer treatment strategies. The research suggests that approaches focusing on corralling stem cell fate, restricting epithelial plasticity, or intervening when tumors lack heterogeneity could dramatically improve therapeutic efficacy. Understanding the timing and conditions under which plasticity occurs may enable more precise treatment scheduling and combination therapies.
The study underscores the critical importance of considering cellular plasticity in therapeutic planning. Rather than viewing resistance as inevitable, researchers can now develop strategies to anticipate and counteract these adaptive mechanisms, potentially transforming outcomes for colorectal cancer patients facing limited treatment options.





