Breakthrough in Xenotransplantation: Pig Kidney Successfully Supports Human Life for 61 Days
A groundbreaking medical study published in Nature reveals that a genetically-modified pig kidney successfully supported life-sustaining functions in a brain-dead human recipient for 61 days. The research demonstrates that minimally gene-edited pig organs can achieve hemodynamic stability, electrolyte balance, and dialysis independence in humans. While the study identified significant immunological challenges, including antibody-mediated rejection that was successfully reversed, it represents a major step forward in addressing the critical shortage of donor organs for end-stage renal disease patients.
In a landmark achievement for transplant medicine, researchers have demonstrated that a genetically-modified pig kidney can successfully support human physiological functions for an extended period. Published in Nature, this groundbreaking study represents a significant advancement in xenotransplantation—the transplantation of organs between different species—offering hope for addressing the critical shortage of donor organs for patients with end-stage renal disease.

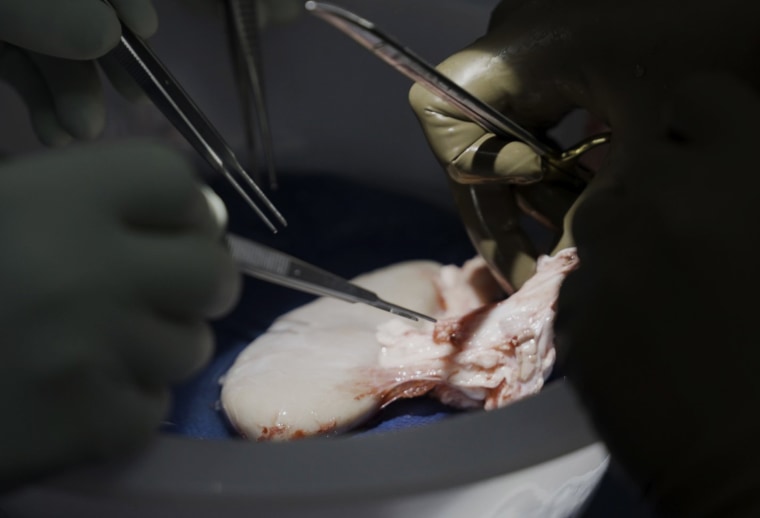
The Xenotransplantation Procedure
The research team from NYU Langone Transplant Institute performed a 61-day transplant of an alpha-Gal knock-out pig kidney and thymic autograft into a nephrectomized brain-dead human recipient. Notably, the procedure used only clinically approved immunosuppression protocols without requiring CD40 blockade or additional genetic modifications. This approach demonstrates that relatively simple genetic engineering may be sufficient to enable cross-species organ compatibility.
Physiological Success and Challenges
The transplanted pig kidney achieved remarkable physiological success, maintaining hemodynamic stability, electrolyte balance, and enabling dialysis independence throughout most of the study period. However, the research also revealed unique immunological challenges not typically seen in human-to-human transplantation. On post-operative day 10, biopsies showed glomerular IgM and IgA deposition, activation of early complement components, and mesangiolysis—a pattern distinct from conventional allotransplantation.
Immunological Hurdles and Solutions
The study encountered a significant challenge on day 33 when an abrupt increase in serum creatinine indicated antibody-mediated rejection. This rejection episode was associated with increased donor-specific IgG antibodies. Importantly, the research team successfully reversed the rejection using plasma exchange, C3/C3b inhibition, and rabbit anti-thymocyte globulin treatment, demonstrating that conventional immunosuppressive strategies can effectively manage xenotransplant rejection.
Future Implications and Research Directions
This research provides the first comprehensive long-term monitoring of pig-to-human kidney xenotransplantation, revealing both the promise and challenges of this approach. The study indicates that pre-existing xenoreactive T cells and induced antibodies to unknown epitopes present significant hurdles, even with substantial immunosuppression. However, the successful 61-day function of a minimally gene-edited pig kidney represents a crucial step toward making xenotransplantation a viable clinical option for the thousands of patients awaiting kidney transplants worldwide.





