Human Liver Assembloids: A Breakthrough in Modeling Periportal Tissue and Disease
Researchers have developed a groundbreaking human liver assembloid system that recapitulates the complex architecture and cellular interactions of periportal liver tissue in vitro. This innovative platform combines patient-derived hepatocyte organoids, cholangiocyte organoids, and portal fibroblasts to create three-dimensional structures that mimic native liver organization. The assembloids maintain patient-specific gene expression, functional hepatocyte characteristics, and can model aspects of biliary fibrosis, offering unprecedented opportunities for personalized medicine, drug development, and disease modeling. This advancement addresses critical limitations in liver research and opens new avenues for studying human liver pathophysiology.
Liver diseases account for over 2 million deaths worldwide annually, yet modeling human liver biology and disease has remained challenging due to species-specific differences and the inability to expand primary human hepatocytes in culture. Traditional models, including rodent systems and cancer cell lines, fail to capture the intricate cellular interactions and patient-specific traits essential for precision medicine. A groundbreaking study published in Nature introduces human periportal liver assembloids—a novel in vitro platform that recapitulates the histological arrangement, gene expression, and cell interactions of native human liver tissue.
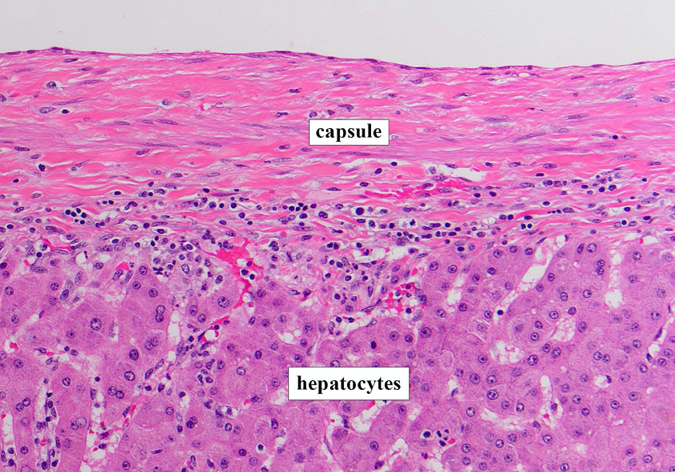
The Challenge of Human Liver Modeling
Despite advances in organoid technology, expanding human adult hepatocytes directly from patient tissue has remained an unmet challenge. Primary hepatocytes cannot be expanded in culture, and existing models—including cancer cell lines and reprogrammed hepatocytes—fail to replicate the three-dimensional bile canaliculus structures observed in native tissue. These limitations have hindered progress in modeling complex disease states and recapitulating patient-specific traits essential for precision medicine and early diagnosis.
The liver's periportal region, where hepatocytes, cholangiocytes (bile duct cells), and portal mesenchyme interact, is particularly difficult to model. This region plays crucial roles in liver function and is often affected in chronic liver diseases. Previous models consisted only of epithelial cells and lacked the ability to fully replicate the cellular interactions and architecture of in vivo adult human liver tissue.
Developing Expandable Human Hepatocyte Organoids
The research team first developed human hepatocyte organoids (h-HepOrgs) from 28 different patients, achieving what had previously been impossible: long-term expansion of human adult hepatocytes directly from fresh patient tissue. Through ingenuity pathway analysis of signaling pathways involved in cancer progression and tissue regeneration, researchers identified that combining WNT activation with YAP signaling enabled serial passaging of h-HepOrgs for over three months.
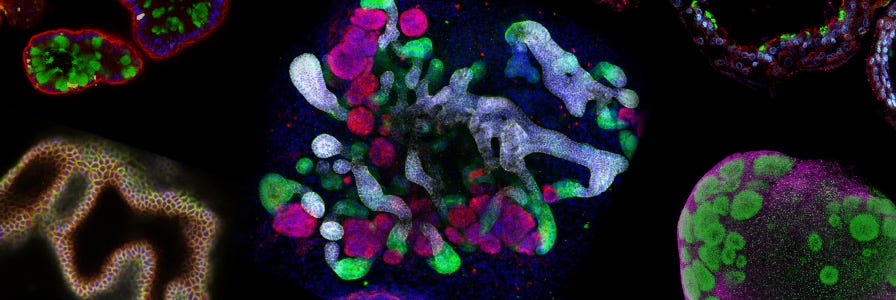
The optimized culture conditions, termed expansion medium 2 (EM2), allowed the generation of expandable h-HepOrgs from 28 patients with 100% efficiency, including samples from cryopreserved hepatocytes. These organoids maintained stable chromosome numbers over time and could be frozen and thawed without loss of expansion capacity, enabling the creation of a living biobank from multiple donors.
Functional Characterization of Hepatocyte Organoids
Expanded h-HepOrgs closely correlated with freshly isolated primary human hepatocytes in gene expression patterns while exhibiting a proliferative signature resembling regenerating tissue after hepatectomy. The organoids expressed hepatocyte markers such as HNF4A and ALB, several apolipoproteins, and cytochromes, albeit at lower levels than freshly isolated hepatocytes.
Upon differentiation in a specialized medium (DM), h-HepOrgs acquired enhanced hepatocyte maturation features: reduced proliferation, higher cytoplasm-to-nucleus ratio, and improved bile canaliculi with thinner, more elongated morphology. Differentiated cells showed increased expression of mature hepatocyte markers, including ALB, apolipoproteins, bile acid transporters, and detoxifying enzymes.
Creating Periportal Liver Assembloids
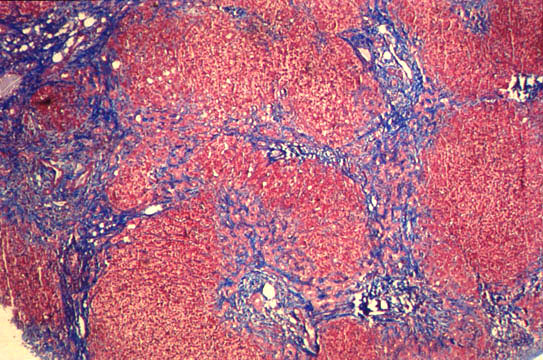
The true breakthrough came when researchers combined these novel patient-derived hepatocyte organoids with primary human portal mesenchyme and human cholangiocyte organoids (h-CholOrgs) from the same patient to generate human periportal assembloids. By quantifying the physiological proportions of the three cell types in vivo (approximately 77% hepatocytes, 15% cholangiocytes, and 8% portal fibroblasts), the team determined optimal ratios for assembloid formation.

The resulting assembloids established key periportal architectural features within three days, with cholangiocytes forming bile duct-like structures containing open lumina in close proximity to portal fibroblasts, both embedded within the hepatocyte parenchyma. This architectural organization, observed in approximately 80% of assembloids across donors, closely mirrored the cellular composition and proportions of native tissue.
Functional Validation of Assembloids
Single-cell RNA sequencing analysis revealed that assembloid cells mostly overlapped with corresponding cells in human liver cell atlases. Hepatocytes, cholangiocytes, and mesenchymal cells from assembloids expressed classical markers of their in vivo counterparts and exhibited functional enrichment patterns similar to human liver tissue.
Notably, periportal assembloids outperformed differentiated h-HepOrgs in portal-specific functions including urea production and gluconeogenesis, while maintaining core hepatocyte functions with albumin secretion matching that of hepatocyte organoids. The periportal microenvironment within assembloids appeared to promote acquisition of a more portal-like hepatocyte identity, with hepatocytes exhibiting higher expression of periportal markers and downregulation of pericentral genes.
Modeling Biliary Fibrosis
The assembloid platform demonstrated remarkable utility in disease modeling when researchers investigated its potential to recapitulate aspects of human biliary fibrosis. By increasing mesenchymal cell numbers while keeping other cell numbers constant, the team generated "fibrotic-like" assembloids that exhibited altered cellular composition reminiscent of fibrotic tissue.
These fibrotic-like assembloids recapitulated transcriptional signatures of diseased human livers, with mesenchyme and cholangiocytes showing increased expression of proteins involved in collagen and matrix deposition processes. Hepatocytes from fibrotic-like assembloids were enriched for inflammatory gene sets while showing negative enrichment for hepatocyte functions such as bile secretion and drug metabolism—patterns mirroring those observed in patients with biliary fibrosis and primary sclerosing cholangitis.
Implications for Research and Medicine
This human liver periportal assembloid system represents a transformative advancement with far-reaching implications. The platform enables investigation of human liver pathophysiology with unprecedented fidelity to native tissue architecture and cellular interactions. By preserving patient-specific signatures, including disease-predisposing genes and metabolic enzyme variations, the system offers powerful opportunities for personalized medicine approaches.
The assembloid technology addresses critical needs in drug development by providing a human-relevant system for toxicity testing and efficacy screening. The ability to model aspects of biliary fibrosis opens new avenues for understanding disease mechanisms and testing potential therapeutics. Furthermore, the demonstration that both expanded and differentiated hepatocyte organoids can engraft and maintain hepatic function following xenotransplantation in mouse models suggests potential applications in cellular transplantation therapy.
Future Directions and Limitations
While the assembloid system represents a significant advancement, researchers note several areas for future development. The current model lacks other mesenchymal cells, immune cells, and portal vasculature (portal vein and hepatic artery), which limits formation of a true periportal triad. Incorporating these components will be crucial to reproduce all aspects of liver physiology and disease.
The modular, "self-organized Lego-like" nature of the assembloid platform provides a unique system to systematically manipulate individual cellular components and dissect how specific microenvironmental signals or cell-cell interactions contribute to human hepatocyte identity and zonation. This capability will enable more precise investigation of liver biology and disease mechanisms.
Conclusion
The development of patient-derived hepatocyte organoids and periportal assembloids marks a paradigm shift in liver research. By overcoming the longstanding challenge of expanding human adult hepatocytes and combining them with other liver cell types to recreate tissue architecture, this platform provides an unprecedented tool for studying human liver biology and disease.
As researchers continue to refine and expand this technology, it holds promise to accelerate drug development, enable early diagnosis, advance personalized medicine, and potentially contribute to cellular transplantation therapies. The human liver assembloid system represents not just a technical achievement but a fundamental advancement in our ability to understand and treat liver diseases that affect millions worldwide.