How Astrocyte CCN1 Maintains Neural Circuit Stability in the Adult Brain
New research reveals that astrocytes, the brain's support cells, actively maintain the stability of neural circuits in adulthood through a protein called CCN1. This study from the Salk Institute demonstrates that astrocyte-secreted CCN1 coordinates the maturation of multiple cell types to restrict plasticity and preserve functional connectivity in the visual cortex. The findings challenge previous assumptions about passive circuit maintenance and open new avenues for understanding brain stability and potential therapies for brain injury recovery.
For decades, neuroscientists have understood that neural circuits exhibit remarkable plasticity during early development but become more stable in adulthood. This stability is crucial for maintaining learned behaviors and functional connectivity, yet the mechanisms underlying this maintenance have remained elusive. Groundbreaking research published in Nature reveals that astrocytes, once considered merely supportive cells, play an active role in stabilizing neural circuits through the secretion of a protein called CCN1.

The study establishes that astrocyte-secreted CCN1 functions as a pro-stability factor that coordinates the maturation state of multiple cell types in the visual cortex. This research not only identifies a specific molecular mechanism for circuit maintenance but also demonstrates that adult neural circuits require ongoing active cues to preserve their functional properties—a paradigm shift in our understanding of brain stability.
The Critical Role of Astrocytes in Neural Circuit Maintenance
Astrocytes have traditionally been viewed as passive support cells in the brain, responsible for nutrient delivery, waste removal, and maintaining the blood-brain barrier. However, recent research has revealed their active participation in regulating synapse formation, function, and plasticity. The new study extends this understanding by demonstrating that astrocytes actively maintain circuit stability in adulthood through specific molecular signals.
The research focused on the mouse visual cortex as a model system due to its well-characterized critical period for binocular circuits. During early development, visual circuits exhibit high plasticity as they refine connections based on visual experience. By adulthood, these circuits become more stable to preserve established visual functions. The study sought to identify how astrocytes regulate this transition from plasticity to stability.
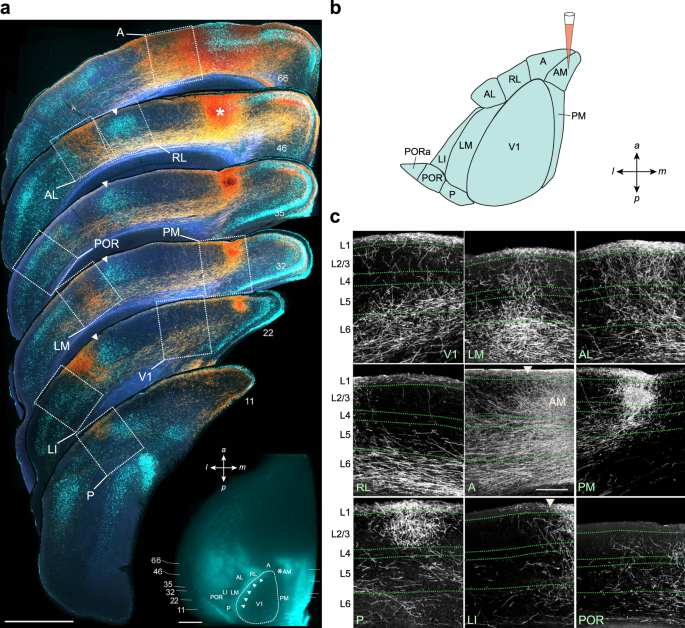
Identifying CCN1 as a Pro-Stability Factor
Using comprehensive transcriptomic analysis combined with electrophysiology and in vivo imaging, researchers identified CCN1 as a key factor upregulated in astrocytes during periods of high stability. CCN1 expression increases in adulthood and decreases when plasticity is induced through experimental manipulations like dark rearing or monocular deprivation.
The protein CCN1, also known as Cyr61, is a secreted matricellular protein containing integrin and heparan sulfate proteoglycan binding sites. While its role in peripheral tissues has been studied in contexts like tumorigenesis and inflammation, its function in the central nervous system remained largely unknown until this research.
Mechanisms of CCN1 Action
Regulating Multiple Cell Types
One of the most significant findings is that CCN1 exerts its effects on multiple cell types simultaneously. The research demonstrates that astrocyte-secreted CCN1 regulates:
- Excitatory neurons: Decreasing excitatory drive onto pyramidal neurons
- Inhibitory neurons: Promoting maturation of parvalbumin-positive interneurons
- Oligodendrocytes: Enhancing differentiation and myelination
- Microglia: Modulating morphological state and reactivity
This coordinated action across different cell types represents a sophisticated mechanism for maintaining circuit stability. The research shows that CCN1 overexpression during the critical period promotes premature maturation of inhibitory neurons and their perineuronal nets—dense extracellular matrices that form around parvalbumin-positive interneurons and correlate with closure of the critical period.
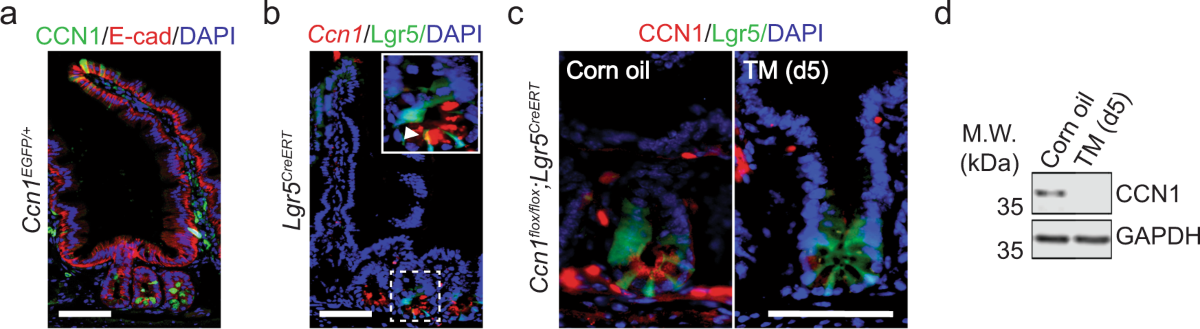
Integrin-Mediated Signaling
The study identified that CCN1's effects on circuit stability require binding to αVβ5 or αVβ3 integrins. A single point mutation (D125A) that renders CCN1 unable to bind these integrins occludes its effects on binocular zone remodeling and oligodendrocyte differentiation. This finding provides crucial insight into the molecular pathways through which CCN1 exerts its pro-stability effects.
Functional Consequences of CCN1 Manipulation
Overexpression During Critical Period
When researchers overexpressed CCN1 in astrocytes during the critical period, they observed restricted plasticity at a time when it would normally be high. Using monocular enucleation to induce remodeling of inputs to the visual cortex, they found that CCN1-overexpressing mice showed much less binocular zone remodeling compared to controls.
Electrophysiological recordings revealed that CCN1 overexpression decreased excitatory drive onto pyramidal neurons while increasing excitatory drive onto inhibitory neurons. This shift in excitation-inhibition balance contributes to circuit stability.
Knockout in Adulthood
Conversely, knocking out astrocyte CCN1 in adult mice destabilized binocular circuits. In vivo two-photon calcium imaging showed that CCN1 conditional knockout mice had altered proportions of contralateral-responsive and binocular-responsive neurons, with increased plasticity after monocular deprivation.
Behavioral testing using the visual cliff assay revealed that CCN1 knockout mice spent more time in the cliff zone and had increased zone transitions, indicating impaired depth perception. This demonstrates that the circuit changes have functional consequences for visual behavior.
Implications for Brain Function and Therapy
The discovery that astrocytes actively maintain circuit stability through CCN1 secretion has several important implications:
- Active Maintenance Paradigm: The findings challenge the view that adult circuit stability is a passive state, instead revealing it as an actively maintained condition requiring ongoing molecular cues.
- Therapeutic Potential: Since both CCN1 and its transcriptional regulator SRF are upregulated after stroke, manipulating CCN1 expression or action could represent a therapeutic strategy to promote remodeling after brain injury.
- Understanding Brain Disorders: Disruptions in circuit stability mechanisms may contribute to neurological and psychiatric disorders characterized by abnormal plasticity or stability.
The research demonstrates that adult visual circuits require ongoing maintenance cues to preserve functional connectivity, and that astrocytes provide these cues through CCN1 secretion. This establishes astrocytes as central orchestrators of the complex cellular interactions that maintain circuit stability.
Future Research Directions
While this study provides crucial insights into how astrocytes maintain circuit stability, several questions remain for future investigation:
- How do other astrocyte-secreted factors interact with CCN1 in regulating circuit stability?
- Are similar mechanisms at work in other brain regions beyond the visual cortex?
- How do experience and environmental factors modulate CCN1 expression and function?
- Could targeting CCN1 pathways help treat conditions involving abnormal circuit stability?

Conclusion
The discovery that astrocyte-secreted CCN1 stabilizes neural circuits in the adult brain represents a significant advance in our understanding of how the brain maintains functional connectivity throughout life. By coordinating the maturation state of multiple cell types—excitatory neurons, inhibitory neurons, oligodendrocytes, and microglia—CCN1 serves as a key molecular signal that restricts plasticity and preserves circuit stability.
This research not only deepens our understanding of fundamental brain mechanisms but also opens new avenues for developing therapies that could promote beneficial plasticity after injury or in neurological disorders. As we continue to unravel the complex interactions between different cell types in the brain, the role of astrocytes as active regulators of neural function becomes increasingly clear and important.





