NAC: The Ribosome's Early Orchestrator of Protein Fate
A groundbreaking study published in Nature reveals the nascent polypeptide-associated complex (NAC) as a master regulator of protein biogenesis. By employing NAC-selective ribosome profiling in C. elegans, researchers discovered NAC's dual role: it acts as an intra-tunnel sensor for extremely short nascent chains and as a chaperone that coordinates translation speed, prevents ribosome collisions, and guides proteins to their correct cellular destinations. This multifaceted function establishes NAC as a critical, early-acting orchestrator of cotranslational proteostasis, fundamentally shaping protein fate from the moment synthesis begins.
The intricate process of building a functional protein is far more sophisticated than a simple assembly line. A landmark study published in Nature has illuminated a critical, early-stage conductor in this cellular symphony: the nascent polypeptide-associated complex (NAC). This research, detailed in the article "NAC controls nascent chain fate through tunnel sensing and chaperone action", reveals NAC as a multifaceted regulator that determines a protein's destiny from the very first moments of its creation.
Unveiling NAC's Multifaceted Role
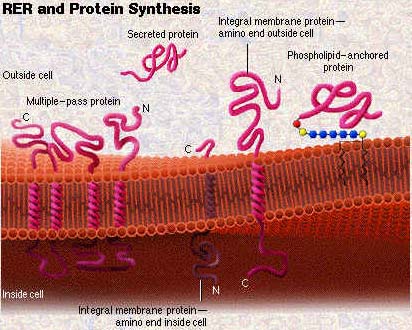
NAC is a conserved, ribosome-bound factor long known to be essential for protein biogenesis, yet its precise mechanisms remained elusive. The new research, led by an international team including scientists from Stanford University, the University of Konstanz, and the Max Planck Institute, employed a powerful technique called NAC-selective ribosome profiling in the model organism C. elegans. This approach allowed them to map thousands of sequence-specific interactions between NAC and nascent polypeptides across the entire proteome.
The findings were profound. NAC engages broadly with hydrophobic and helical motifs in proteins destined for diverse locations, including the cytosol, nucleus, endoplasmic reticulum (ER), and mitochondria. This indicates its role is not limited to a single pathway but is a universal feature of protein synthesis.
A Sensor Inside the Tunnel
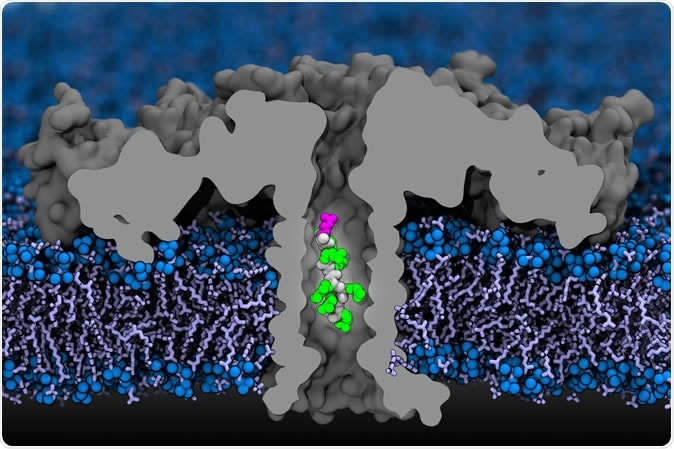
One of the most unexpected discoveries was NAC's ability to act as an intra-tunnel sensor. The researchers found that NAC can engage ribosomes while the nascent polypeptide chain is still extremely short and physically located inside the ribosome's exit tunnel. This interaction is sequence-specific, meaning NAC can "read" the emerging amino acid code and respond accordingly, making it one of the earliest decision points in a protein's life.

Chaperone Action and Kinetic Control
Beyond sensing, NAC functions as a chaperone. Its initial binding induces an early slowdown in translation elongation. This kinetic control serves a vital purpose: it tunes the flow of ribosomes along the mRNA and helps prevent damaging ribosome collisions. By modulating the speed of synthesis, NAC provides crucial time and a protective environment for the nascent chain to begin folding correctly, particularly for aggregation-prone segments featuring amphipathic helices.
Orchestrating Cellular Destination
The study further clarifies how NAC contributes to protein targeting. It supports the biogenesis of mitochondrial membrane proteins and ER targeting by facilitating the early recognition of specific signal sequences and transmembrane domains. In essence, NAC helps interpret the protein's "zip code" early in synthesis, setting it on the correct path to its final cellular location.
This early intervention is key to proteostasis—the cell's careful balance of protein production, folding, and degradation. By shielding vulnerable regions and guiding localization, NAC prevents misfolding and aggregation from the outset, promoting efficient cytonuclear folding and overall cellular health.
Conclusion: A Foundational Regulator
This research fundamentally redefines our understanding of NAC. It is not merely a passive bystander but an active, early-acting orchestrator of cotranslational proteostasis. Through its dual capabilities of intra-tunnel sensing and chaperone-mediated kinetic control, NAC acts as a central hub that coordinates translation speed, folding efficiency, and subcellular targeting based on the nascent chain's sequence. These findings, as published in Nature, highlight a sophisticated layer of regulation that ensures proteins are born correctly, directly influencing cellular function and resilience.